Navigating the Chemical Landscape: A Comprehensive Guide to Mole Map Chemistry
Related Articles: Navigating the Chemical Landscape: A Comprehensive Guide to Mole Map Chemistry
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Navigating the Chemical Landscape: A Comprehensive Guide to Mole Map Chemistry. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: Navigating the Chemical Landscape: A Comprehensive Guide to Mole Map Chemistry
- 2 Introduction
- 3 Navigating the Chemical Landscape: A Comprehensive Guide to Mole Map Chemistry
- 3.1 Understanding the Mole Map
- 3.2 Applications of the Mole Map
- 3.3 Examples of Mole Map Applications
- 3.4 FAQs Regarding Mole Map Chemistry
- 3.5 Tips for Effective Mole Map Utilization
- 3.6 Conclusion
- 4 Closure
Navigating the Chemical Landscape: A Comprehensive Guide to Mole Map Chemistry
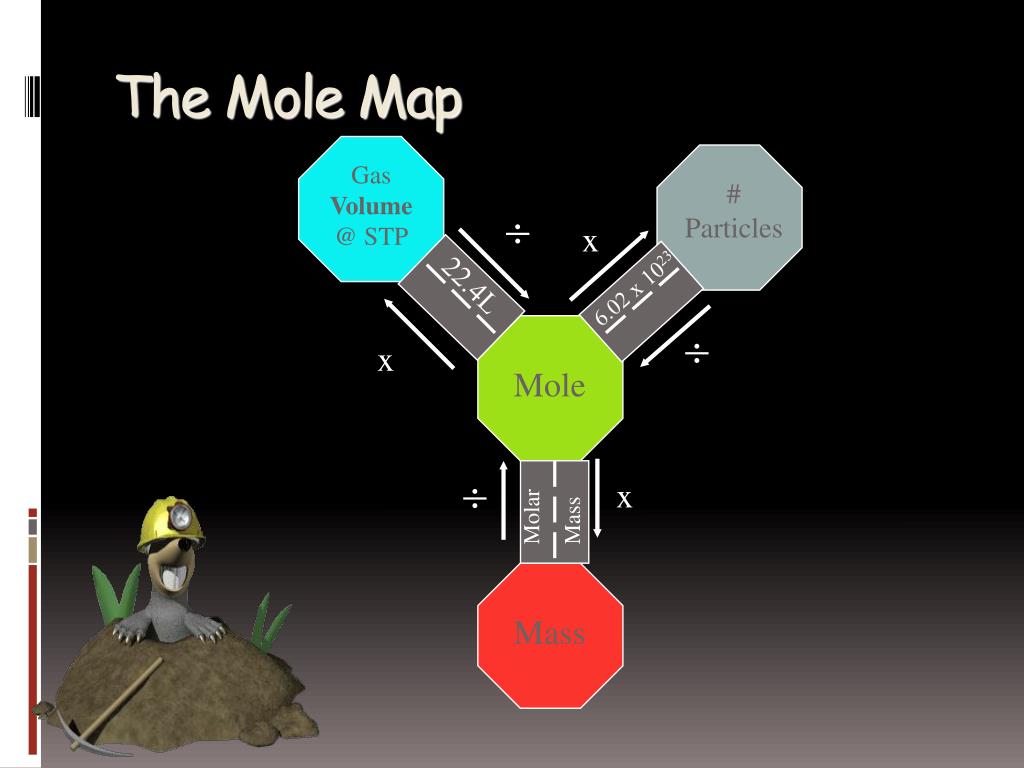
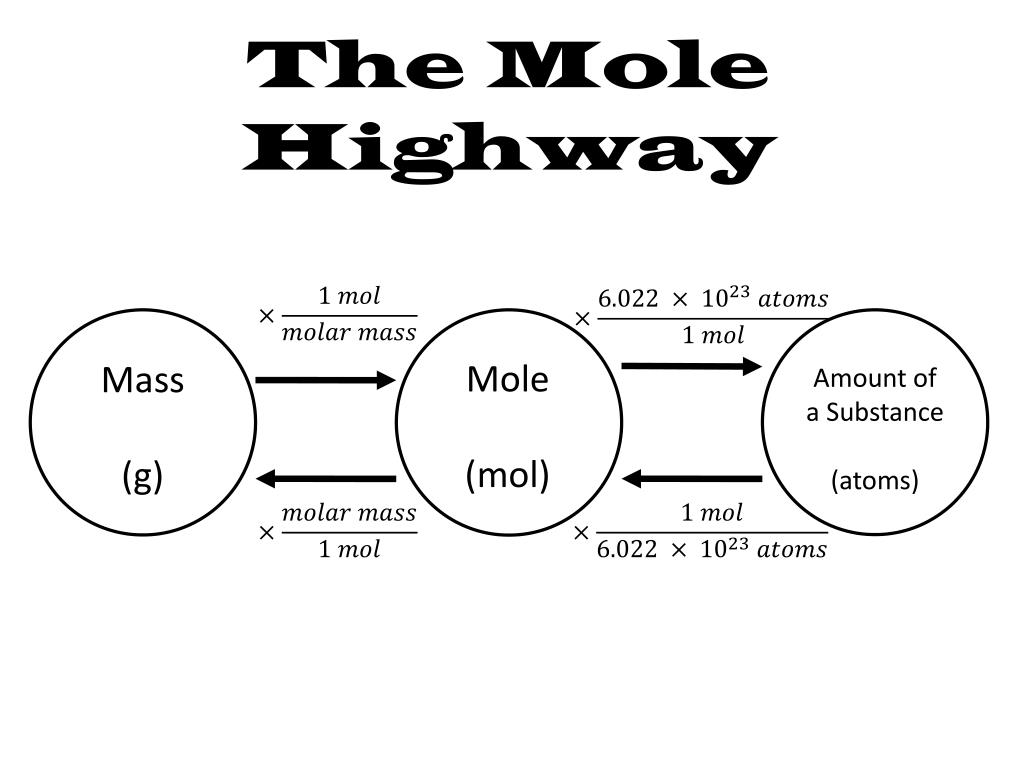
Chemistry, the study of matter and its properties, relies heavily on the concept of the mole. This fundamental unit, representing a specific number of particles (6.022 x 10^23), provides a standardized way to measure and compare amounts of substances. However, navigating the complex relationships between moles, mass, volume, and other chemical quantities can be challenging. This is where the "mole map" comes in, a visual tool that simplifies and clarifies these connections.
Understanding the Mole Map
The mole map, also known as the "mole triangle" or "mole roadmap," is a diagram that visually represents the relationships between various chemical quantities. It typically includes the following key components:
- Moles (mol): The fundamental unit of measurement in chemistry, representing a specific number of particles (Avogadro’s number).
- Mass (g): The amount of matter in a substance, measured in grams.
- Volume (L): The space occupied by a substance, measured in liters (for gases) or milliliters (for liquids).
- Molar Mass (g/mol): The mass of one mole of a substance, determined by the atomic masses of its constituent elements.
- Density (g/mL or g/L): The ratio of mass to volume, indicating the concentration of matter in a substance.
- Concentration (mol/L): The amount of solute dissolved in a given volume of solvent, measured in moles per liter.
The mole map visually connects these quantities through arrows, indicating the conversion factors required to move between them. For example, an arrow pointing from "moles" to "mass" implies that multiplying the number of moles by the molar mass will yield the mass of the substance.
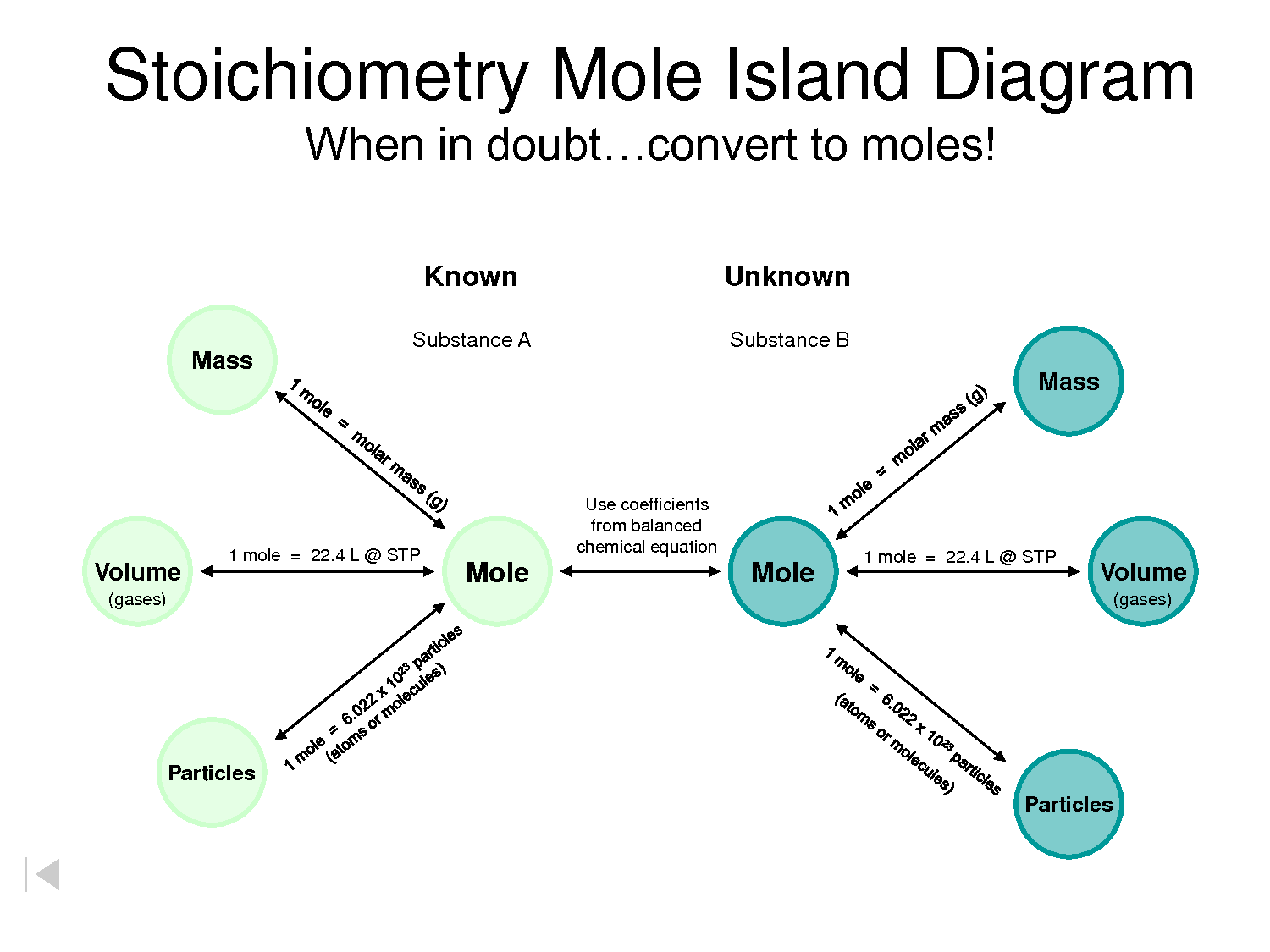
Applications of the Mole Map
The mole map serves as a valuable tool for solving a wide range of chemical problems, including:
- Stoichiometry Calculations: Determining the amounts of reactants and products involved in chemical reactions.
- Solution Preparation: Calculating the mass of solute needed to prepare a solution of specific concentration.
- Gas Law Calculations: Relating the volume, pressure, and temperature of gases using the ideal gas law.
- Titration Analysis: Determining the concentration of an unknown solution by reacting it with a solution of known concentration.
By understanding the relationships between various chemical quantities, the mole map empowers students and professionals to solve complex chemical problems with greater ease and accuracy.
Examples of Mole Map Applications
-
Stoichiometry Calculations: Consider the following balanced chemical equation:
2H₂ + O₂ → 2H₂O
This equation indicates that 2 moles of hydrogen gas (H₂) react with 1 mole of oxygen gas (O₂) to produce 2 moles of water (H₂O). Using the mole map, we can calculate the mass of water produced when 5 grams of hydrogen gas reacts completely.
- Step 1: Convert the mass of hydrogen gas to moles using the molar mass of hydrogen (2 g/mol).
5 g H₂ x (1 mol H₂ / 2 g H₂) = 2.5 mol H₂ - Step 2: Use the mole ratio from the balanced equation to find the moles of water produced.
2.5 mol H₂ x (2 mol H₂O / 2 mol H₂) = 2.5 mol H₂O - Step 3: Convert the moles of water to grams using the molar mass of water (18 g/mol).
2.5 mol H₂O x (18 g H₂O / 1 mol H₂O) = 45 g H₂O
Therefore, 5 grams of hydrogen gas will produce 45 grams of water.
- Step 1: Convert the mass of hydrogen gas to moles using the molar mass of hydrogen (2 g/mol).
-
Solution Preparation: To prepare a 0.5 M solution of sodium chloride (NaCl) in 500 mL of water, we can use the mole map to calculate the mass of NaCl needed.
- Step 1: Convert the volume of the solution to liters.
500 mL x (1 L / 1000 mL) = 0.5 L - Step 2: Multiply the concentration by the volume to find the number of moles of NaCl.
0.5 mol/L x 0.5 L = 0.25 mol NaCl - Step 3: Convert the moles of NaCl to grams using the molar mass of NaCl (58.44 g/mol).
0.25 mol NaCl x (58.44 g NaCl / 1 mol NaCl) = 14.61 g NaCl
Therefore, 14.61 grams of NaCl are needed to prepare a 0.5 M solution in 500 mL of water.
- Step 1: Convert the volume of the solution to liters.
FAQs Regarding Mole Map Chemistry
1. What is the significance of Avogadro’s number in the mole map?
Avogadro’s number (6.022 x 10^23) represents the number of particles (atoms, molecules, ions) in one mole of a substance. This constant forms the basis for the mole concept and connects the microscopic world of atoms and molecules to the macroscopic world of grams and liters.
2. How does the mole map help in understanding the ideal gas law?
The ideal gas law (PV = nRT) relates the pressure (P), volume (V), temperature (T), and number of moles (n) of a gas. The mole map highlights the relationship between moles and volume, allowing us to convert between these quantities using the ideal gas constant (R).
3. Can the mole map be used for all types of chemical calculations?
While the mole map is a versatile tool, it is primarily designed for calculations involving moles, mass, volume, and other related quantities. It may not be directly applicable to calculations involving other chemical concepts like enthalpy, entropy, or Gibbs free energy.
4. What are the limitations of using the mole map?
The mole map provides a simplified representation of chemical relationships and may not always account for specific conditions or complexities. For example, it does not consider the effects of non-ideal behavior in gases or the presence of impurities in substances.
5. How can I improve my understanding of the mole map and its applications?
Practice is key to mastering the mole map and its applications. Work through numerous examples, solve practice problems, and seek guidance from teachers or tutors. Visualizing the relationships between quantities and understanding the underlying principles will enhance your grasp of the concept.
Tips for Effective Mole Map Utilization
- Visualize the Connections: Focus on the arrows and their directions, understanding the conversion factors involved.
- Start with the Known: Identify the given quantity and use the mole map to navigate to the desired quantity.
- Write Out the Units: Ensure that the units cancel out correctly during calculations, preventing errors.
- Check for Consistency: Verify that the units of all quantities are consistent throughout the calculation.
- Practice Regularly: Solve numerous problems to reinforce your understanding and build confidence.
Conclusion
The mole map is a powerful tool that simplifies the complex relationships between various chemical quantities. By understanding its structure and applications, students and professionals can effectively solve a wide range of chemical problems, from stoichiometry calculations to solution preparation. By consistently practicing and visualizing the relationships between quantities, mastering the mole map can significantly enhance your understanding and proficiency in chemistry.





Closure
Thus, we hope this article has provided valuable insights into Navigating the Chemical Landscape: A Comprehensive Guide to Mole Map Chemistry. We thank you for taking the time to read this article. See you in our next article!